Advancing Patient Care through Active Management of Adverse Drug Events and Drug Information Services
Read the Magazine in PDF
Abstract :
This report discusses the importance of actively managing and capturing adverse drug events and drug information services in a healthcare setting. The pharmacy department’s Drug Information Center (DIC) is a referral service for medication queries or concerns. Inadequate monitoring of adverse drug events can have significant health implications, and accurate documentation can effectively reduce the incidence of such reactions. The report describes the implementation of a robust monitoring system and the proactive identification of adverse drug events, which have reduced the length of hospital stays for many patients.
Organizational Profile:
Shri. B. Nagi Reddi was a versatile personality honoured with the prestigious Dada Saheb Phalke Award for his invaluable contributions to the Indian film industry. He founded Vijaya Vauhini Studios, a renowned film production destination in India, and Prasad Process, one of India’s largest printing presses that published popular children’s magazines like “Chandamama”. Despite his accomplishments, Shri. B. Nagi Reddi’s ultimate goal was to provide affordable medical services to society. This vision led to the establishment of Vijaya Hospital in 1972, one of Chennai’s first multi- speciality hospitals, starting with 30 beds and now expanding to over 750 beds under the Vijaya Medical and Educational Trust (VMET) umbrella, which includes Vijaya Hospital (VH), Vijaya Health Centre (VHC), Vijaya Heart Foundation (VHF), and Vijaya Eye Foundation.
Introduction:
Healthcare professionals can encounter adverse drug events (ADEs) and adverse drug reactions (ADRs) during treatment, which the drug may not necessarily cause. The pharmacy department provides a Drug Information Center (DIC) service, which acts as a referral service by directing professionals to the best available resources to address queries or concerns. This service is specifically designed to meet the needs of healthcare professionals. Moreover, inadequate monitoring of adverse drug events can have significant health implications. However, maintaining accurate documentation of these events can effectively reduce the incidence of such reactions. To address the issue, we have implemented a robust monitoring system to ensure the quality of medication and detect counterfeit drugs. Our team of experts captures even minor signs and symptoms that patients may experience.
The clinical pharmacology team actively monitors cases from inpatient and outpatient services and thoroughly analyses any events or reactions. This includes a comprehensive review of relevant literature, batch checks, and causality assessments.
Patients and the general public can contact the drug information centre in person or through designated email or WhatsApp to discuss any newly developed symptoms.
Outcome:
Implementing a comprehensive Drug Information Center (DIC) by our pharmacy team has proven to be an effective solution for ensuring patient safety and providing valuable education to the public regarding drug-related matters. The DIC plays a crucial role in capturing and addressing any new or chronic symptoms that may arise after medication initiation.
A systematic process has been established to identify and address potential adverse drug events (ADEs). When a drug is suspected of causing a symptom, the case is escalated to a consultant for further investigation. An Adverse Drug Event (ADE) Form is then raised, supported by relevant evidence, to thoroughly document and analyze the incident.
To ensure the sustainability of this improvement, the following actions have been implemented to lock in the positive outcomes:
- Strict monitoring of drugs: A rigorous monitoring system has been put in place to track and assess the safety and efficacy of medications used in patient care. This includes regular drug profile reviews, monitoring potential side effects, and staying updated with the latest research and clinical guidelines.
- Regular monitoring of patients’ daily activities: Continuous monitoring of patients’ daily activities helps promptly identify any changes in symptoms, side effects, or adverse reactions. This proactive approach allows for early intervention and timely adjustments in medication regimens if needed.
- Answering/responding to queries within 24 hours: Efficient communication and responsiveness are crucial in maintaining patient satisfaction and safety. Therefore, all questions and concerns received through the Drug Information Center are addressed and responded to within 24 hours. This ensures that patients and the public receive accurate and timely medication information.
In addition to the successful implementation of the DIC and the systematic approach to addressing ADEs, the improvement initiative has been extended to identify adverse drug events, Focusing on polypharmacy-related incidents proactively. By proactively identifying ADES, healthcare providers can take appropriate measures to prevent or minimize patient harm. This proactive approach has significantly reduced the length of hospital stays for many patients, as potential drug-related complications are detected early, leading to timely interventions and improved outcomes.
The success of cloning this improvement lies in replicating the established processes and practices across different healthcare settings and departments. By leveraging the lessons learned and best practices from the initial implementation, other healthcare facilities and departments can adopt similar strategies to enhance patient safety, reduce hospital stays, and promote more effective management of medication-related issues.
From August 2021 to June 2022, the drugs commonly associated with adverse drug events (ADES) have been identified.
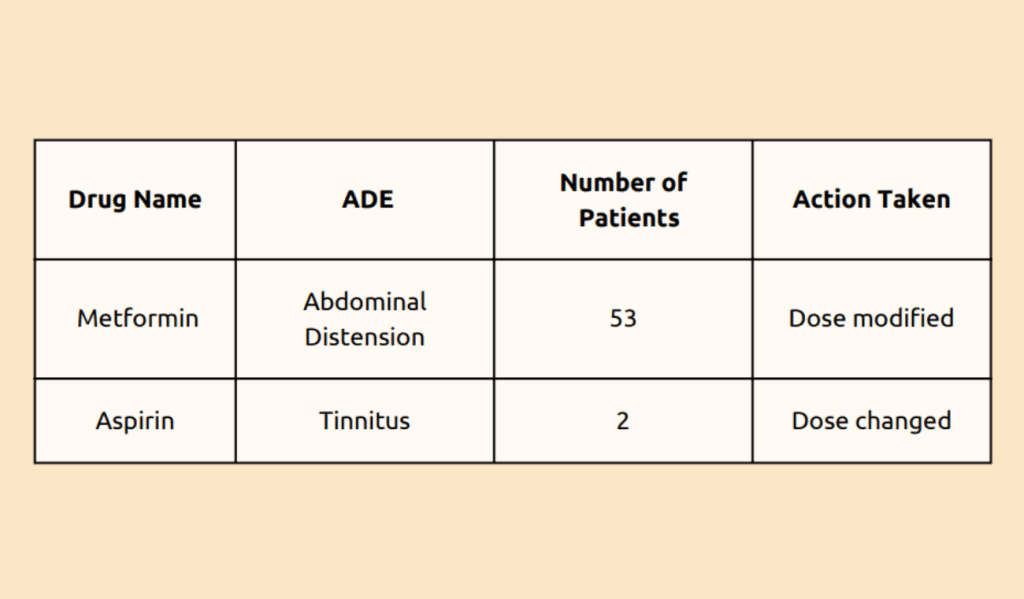
In all cases, the drug was either changed or the dose modified to address the ADEs. Lastly, monitoring the causality of a drug when the public reports an adverse drug reaction or event outside of the hospital can be challenging. Other factors that make monitoring difficult include the dietary and lifestyle modifications of the public.
Conclusion
Vijaya Hospital has taken a proactive approach to managing and capturing adverse drug events and drug information services. The Pharmacy team’s comprehensive Drug Information Centre (DIC) ensures complete patient safety and educates the public regarding various aspects of drugs. The hospital’s team of experts captures even minor signs and symptoms that patients may experience and thoroughly analyses any events or reactions. The report highlights the importance of accurately documenting adverse drug events and describes the hospital’s monitoring system to detect counterfeit drugs. Overall, Vijaya Hospital’s approach to managing and capturing adverse drug events has improved patient outcomes and reduced hospital stays.