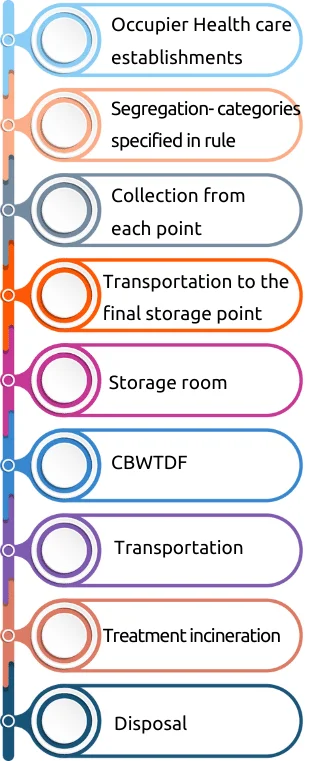
Transportation and Treatment of BMW
Read the Magazine in PDF
Abstract
The management of biomedical waste is integral to healthcare operations, addressing waste generated during diagnosis, immunization, research, treatment, and various healthcare activities. This comprehensive review aims to outline the landscape of biomedical waste management practices prevalent in healthcare facilities, encompassing various aspects from waste generation to disposal and the latest technological interventions. The article delineates the sources of biomedical waste, primarily stemming from healthcare establishments, veterinary hospitals, research organizations, medical camps, and pharmaceutical entities. While biomedical waste accounts for 15% of a hospital’s total waste, its hazardous nature demands stringent management practices. A critical emphasis is placed on the imperative of proper waste segregation. While 85% of hospital waste is non-infectious, the remaining 15%, consisting of infectious and hazardous waste, when combined, escalates the risk, necessitating segregation at the source for distinct treatment and disposal. The Bio-Medical Waste (Management and Handling) Rules formulated in 1998 by the Ministry of Environment and Forests (MoEF), subsequently amended in 2000 and 2016, establish guidelines for healthcare establishments and operators, mandating responsibility for environmental protection and monitored by pollution control boards. Further, the article details the responsibilities associated with biomedical waste management, the categorization of waste, and the final storage points ensuring proper sealing, segregation, and disposal. Innovative technological interventions such as barcoding, GPS tracking, and online monitoring systems are highlighted, showcasing how healthcare establishments like Ken Biolinks have leveraged technology to improve waste inventory recording, transportation tracking, and treatment processes. Treatment methods, including incineration, atomized autoclaves, and effluent treatment processes, are discussed in-depth, shedding light on their respective capacities and functionalities. Additionally, newer technologies like sludge filter presses and online emission monitoring systems are introduced, demonstrating their roles in waste volume reduction and real-time oversight. This article serves as a comprehensive guide, illustrating the multifaceted landscape of biomedical waste management, encompassing regulations, responsible practices, technological advancements, treatment methodologies, and emerging innovations. It emphasizes the critical need for robust waste management practices in healthcare facilities to protect public health and preserve the environment.
Introduction
Biomedical waste means waste, that is generated during diagnosis, immunisation, research or treatment of human beings or animals or research activities pertaining thereto or in the production or testing of biological or health camps. In short, it is nothing but DIRT(S):
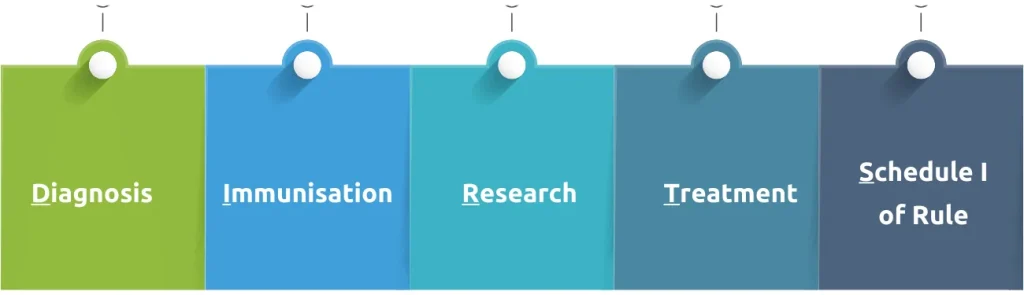
Sources of BMW generation
1. Health care establishments
2. Veterinary hospitals
3. Research organizations
4. Medical camps
5. Pharmaceutical companies/distributors
Is BMW-Hazardous?
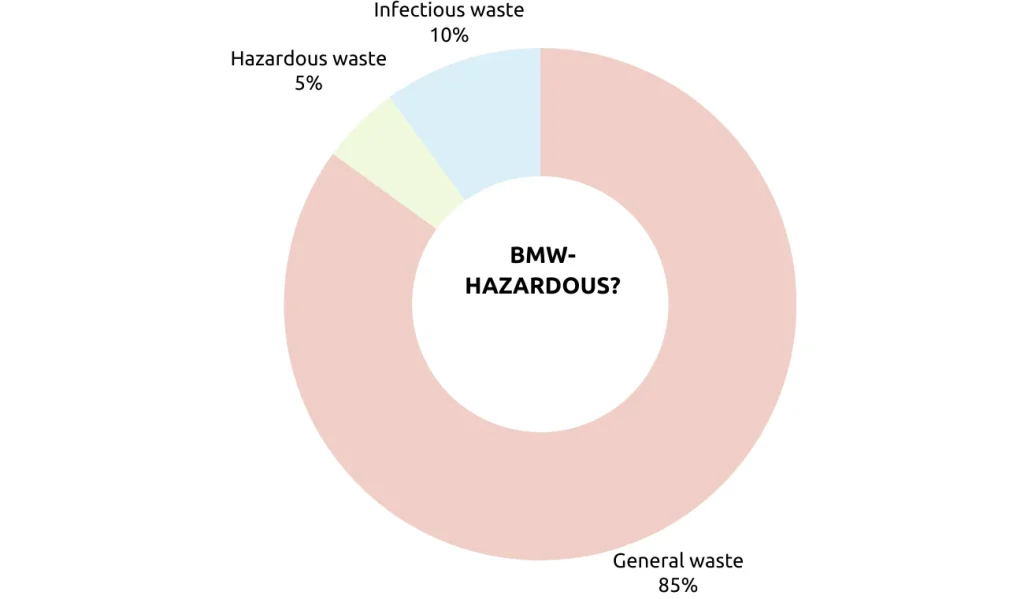
Biomedical waste (BMW) is hazardous due to its potential risk to public health and the environment. While only constituting about 15% of a hospital’s total waste, this fraction contains infectious and hazardous materials, including biomedical sharps, contaminated dressings, pathological wastes, and chemical substances like expired drugs or disinfectants.
When not managed properly, this type of waste can pose severe risks. If infectious or hazardous biomedical waste mixes with non-infectious waste, it can render the entire waste stream hazardous. Therefore, segregation of biomedical waste at its source is crucial to ensure safe handling, treatment, and disposal, preventing any potential harm to individuals, healthcare workers, and the environment.
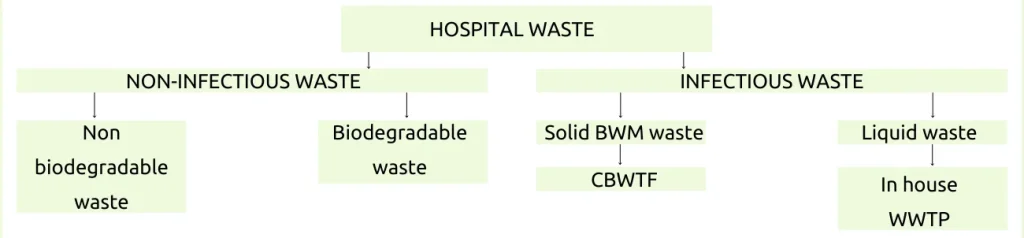
Types of waste generated in a health care establishment
Why BMW waste segregation is more important?
- 85% of hospital waste is non-infectious (Kitchen waste, paper)
- 10% of Hospital waste is highlighted as infectious (dressing, anatomical wastes, blood bags)
- 5% is non-infectious but hazardous (chemical drugs and mercury) When this 15% of the hospital infectious material is mixed with 85%, then all the 100$ waste becomes hazardous and infectious, hence segregation should be done at the source and treated separately before it is disposed of.
Biomedical waste management rules, 2016
Formulated in 1998 by MoEF
- Amended in 2000
- Modified in 2016
- Makes the occupier (Healthcare establishment and operator (common BMW treatment & Disposal facility)) responsible for protecting the environment from hazards, monitored by the state and central pollution control board.
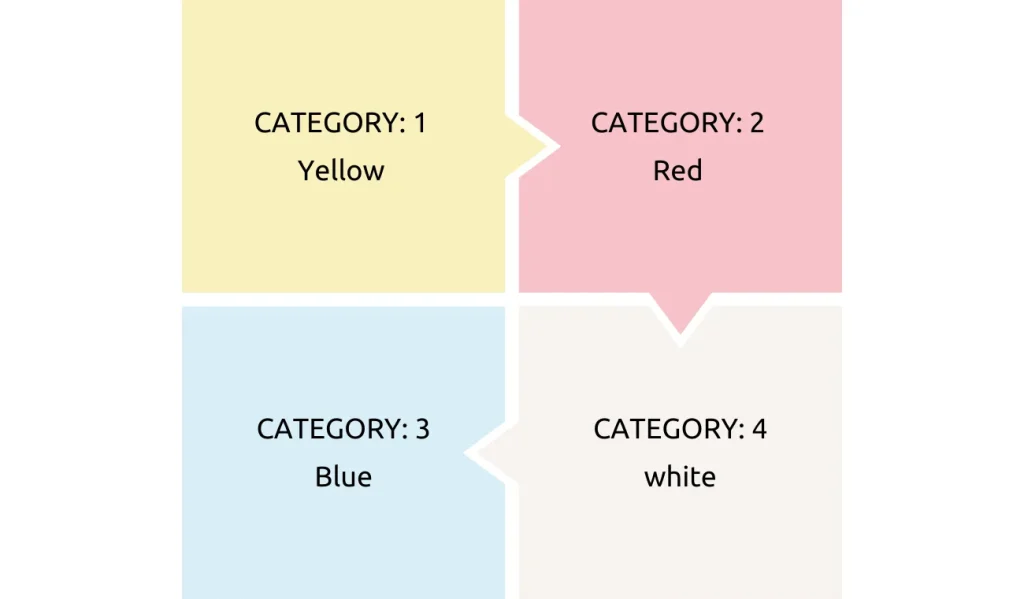
Biomedical waste categories
Final storage Point
- The biomedical waste should be stored in a final storage room. It has to be exclusively used for this purpose. It has to be exclusively used for this purpose. It should be cleaned and disinfected regularly.
- The bags should be sealed properly using tags.
- The seated yellow and red bags should be placed in the respective containers at the final storage point.
- The sharp-metals, glassware and metallic implants to be kept in respective containers.
Safety measures (as per BMW rules) at ken biolinks;
- Recording of waste inventory
- Bar coding of bags
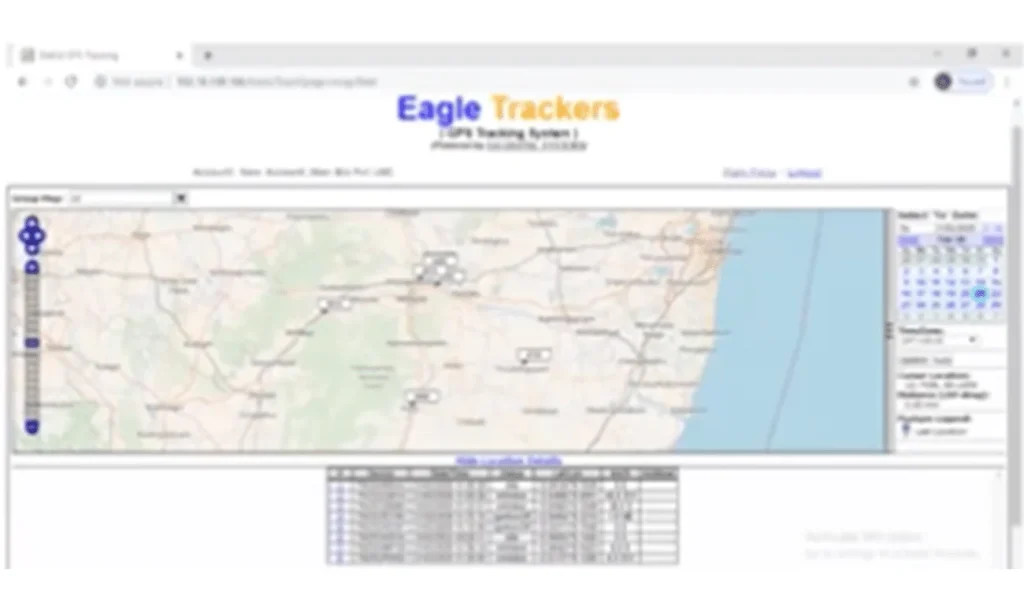
- GPS tracking of vehicles carrying such waste
- Continuous online monitoring of emission system at CBWTF
Key bar stickers

1 Biomedical waste facility, name
2 Address
3 Phone No
4 Barcode
5 Barcode reference no
6 Hospital Name
Key bar stickers on linen

Ken biolinks have bar code scanning mobile app for better tracking of the BMW
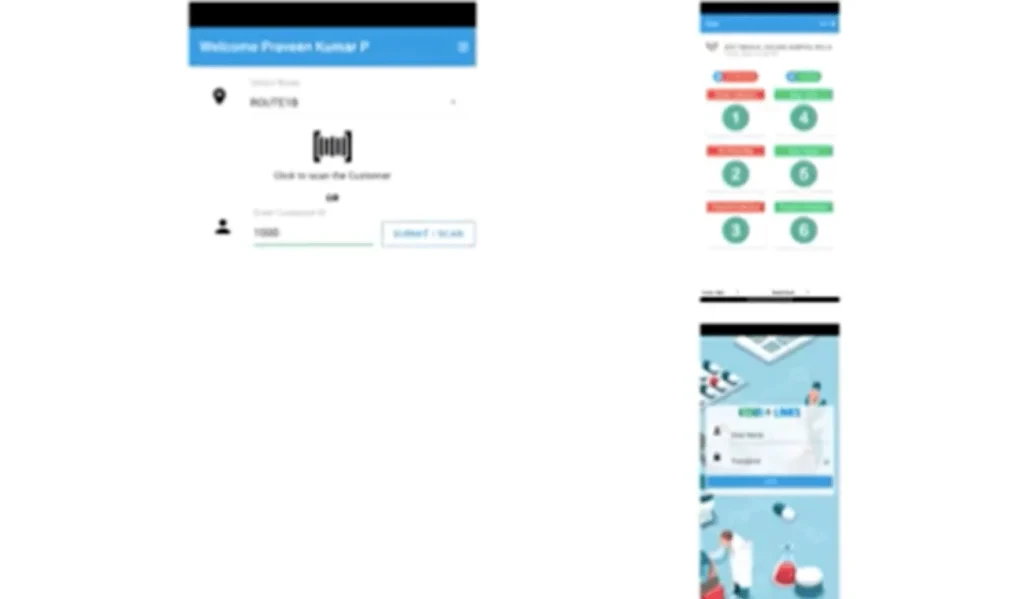
Bar code web portal giving all the details of each hospital to track all the records.
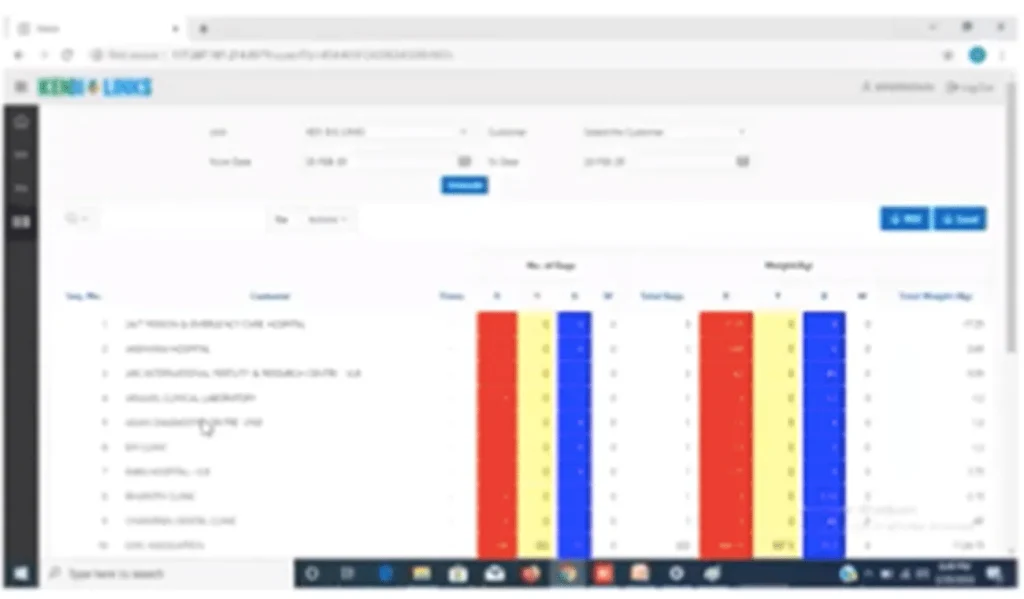
BMW collection
1. Biomedical waste collection and scanned @ HCEs

2. Collecting BMW weight details entering into system

3. Transportation are equipped with GPS: As per biomedical waste management rules 2016 we enabled GPS in all out vehicles. And these service vehicles, which help to track the vehicle in real time.

Treatment process at facility
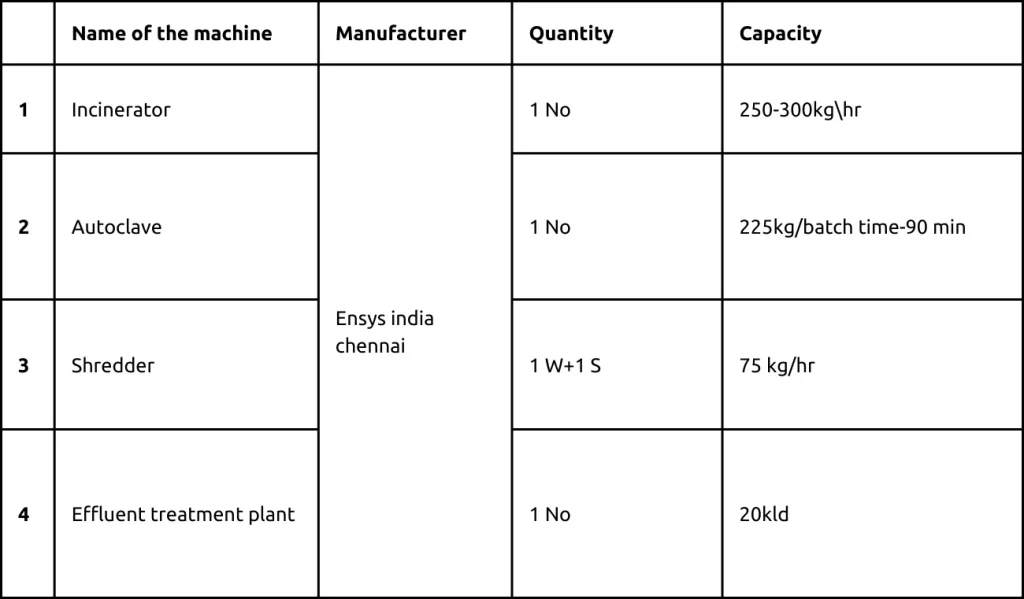
Incinerator

Capacity: 250-300kgs/hour Make : ENSYS INDIA, Chennai
Machinery and capacity
APC system

Atomized Autoclave
The non-incinerable bio-medical waste collected in red bags and punctures filled containers, will be subjected to steam sterilization using vacuum autoclave.
In this process, the biomedical waste will be completely disinfected and decontaminated. The vacuum autoclave will reach a temperature level up to 121C and a pressure level of 20 PSI.
The operating cycle of each batch will be 45minutes.
The autoclave will also have automatic programmable logic controller) data recording instruments so that the temperatures and other relevant information will be available on line and recorded for future reference.

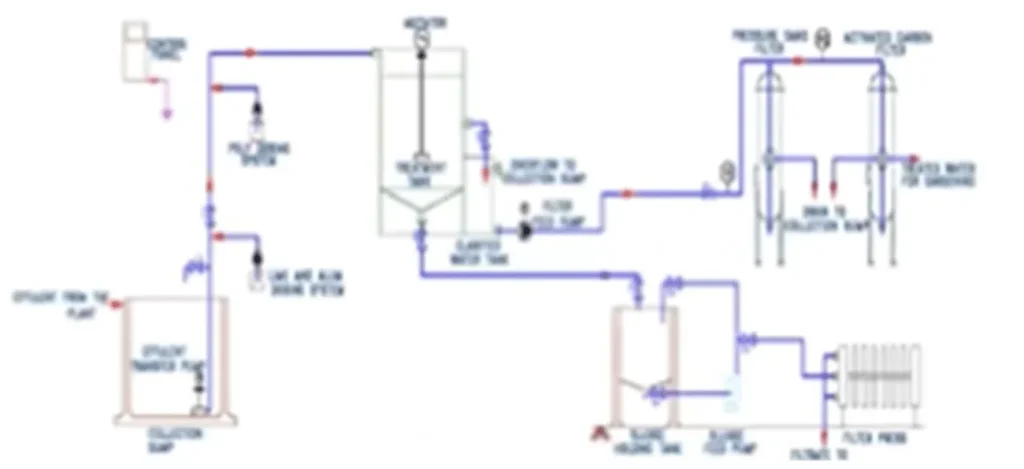
Effluent treatment process
Effluent treatment involves a series of processes designed to purify and remove contaminants from wastewater before its discharge into the environment. The treatment process typically includes several stages:
1. Screening: The first step involves the removal of large objects, debris, and solids from the wastewater using screens or filters. This helps prevent damage to downstream equipment and facilitates subsequent treatment processes.
2. Primary Treatment: In this stage, physical processes such as sedimentation or settling tanks are used to allow suspended solids to settle at the bottom of the tank, forming sludge. This process helps remove a significant portion of solids and some organic matter.
3. Secondary Treatment: This step involves biological processes where microorganisms break down organic matter that escaped the primary treatment. Common secondary treatment methods include activated sludge processes, biological filters, or constructed wetlands. These processes aim to further reduce the organic content and pollutants in the effluent.
4. Tertiary Treatment: Tertiary treatment focuses on the removal of remaining impurities, including nutrients (such as nitrogen and phosphorus) and trace contaminants. Advanced filtration techniques like sand filtration, membrane filtration, or chemical treatments (like chlorination or ozonation) are employed in this stage to achieve high-quality effluent.
5. Disinfection: Before discharge, the treated wastewater undergoes disinfection to kill pathogens and microbes. Common disinfection methods include chlorination, UV irradiation, or zonation.
6.Sludge Treatment: Any sludge generated during the treatment process needs proper handling. Sludge can undergo further treatment steps like digestion, dewatering using filter presses or centrifuges, and sometimes incineration or landfilling.
The overall effluent treatment process aims to reduce the organic load, remove pollutants, and ensure that the discharged water meets regulatory standards, minimizing its environmental impact on receiving bodies of water or land areas.


Sludge

Disposal at facility

Shredder for compaction/physical deformation of plastics before end disposal. Incineration ash filled in HDPE drums and stored at closed shed.


New technologies
Sludge Filter Press- a Sludge after filter press
A sludge filter press is a specialized apparatus used in wastewater treatment to dewater sludge, reducing its volume and facilitating its disposal. This equipment operates by applying pressure to slurry or sludge material, forcing the separation of solids from liquids.
Key components and functions of a sludge filter press include:
- Filter Plates: These plates, typically made of reinforced polypropylene or similar materials, are arranged in a stack within the press. The sludge is pumped into these chambers.
- Pumping System: The sludge is introduced into the filter press using a pumping system that delivers the material under pressure.
- Filtration Process: Once inside the press, the sludge material is subjected to high pressure, which forces the liquid (filtrate) to pass through the filter cloth while retaining the solid components.
- Dewatering: As the pressure is applied, the sludge undergoes dewatering, reducing its moisture content and volume.
- Discharge of Solid Cake: The separated solids form a filter cake, which is discharged from the press, usually in batches, ready for disposal.
- The sludge filter press plays a crucial role in wastewater treatment plants by efficiently managing and minimizing the volume of sludge produced during the treatment process, allowing for easier handling and disposal of the solid waste.

Online emission and monitoring system
An online emission and monitoring system for biomedical waste management (BMW) would involve a digital platform or software designed to oversee and track various aspects of biomedical waste disposal. This system could include real-time monitoring of waste generation, segregation, collection, treatment processes, and disposal methods within healthcare facilities.
Key features might encompass:
1.Real-time Tracking: Monitoring the quantity and type of biomedical waste generated across different departments or units within a healthcare facility.
2.Segregation and Categorization: Ensuring proper segregation and categorization of biomedical waste according to color-coded categories and regulatory guidelines.
3.Treatment Monitoring: Overseeing the treatment processes, whether through incineration, autoclaving, chemical disinfection, or other approved methods, to verify compliance and effectiveness.
4.Transportation Oversight: Tracking the movement of waste from healthcare facilities to treatment and disposal sites, ensuring adherence to safety protocols during transit.
5.Compliance and Reporting: Facilitating compliance with regulatory standards by generating reports, maintaining records, and alerting authorities about any deviations or irregularities.
6. Environment Impact Assessment: Providing data on the environmental impact of waste management practices, including emissions, to facilitate better environmental management.
Such a system, when integrated into biomedical waste management practices, can streamline operations, enhance transparency, and ensure adherence to guidelines, ultimately promoting more effective and environmentally responsible waste disposal.