Regulatory framework of biomedical waste management in India
Read the Magazine in PDF
Abstract
The article explores the comprehensive regulatory framework governing biomedical waste management in India, primarily anchored by the Biomedical Waste Management Rules, 2016. It delves into the guidelines designed to ensure safe handling, treatment, and disposal of biomedical waste to mitigate health and environmental risks. The framework encompasses guidelines for waste segregation, collection, treatment, and disposal, emphasizing compliance and stringent measures to protect public health and the environment. Additionally, the piece outlines significant amendments, highlights key differences between earlier rules, and addresses the penalties associated with non-compliance.
Introduction
The regulatory framework governing biomedical waste management in India is a structured set of guidelines and rules designed to ensure safe and systematic handling, treatment, and disposal of biomedical waste. These regulations aim to mitigate health and environmental risks posed by medical waste generated in healthcare facilities. Governed primarily by the Biomedical Waste Management Rules, 2016, these guidelines delineate procedures for waste segregation, collection, treatment, and disposal. The framework establishes protocols for waste categories, color-coded segregation, and the responsibilities of healthcare facilities, emphasizing compliance and stringent measures to protect public health and the environment.
BMW rules comes under?
1. Ministry of health and family welfare
2. Ministry of environment, forest & climate change
3. Ministry of resources
4. Ministry of water resources.
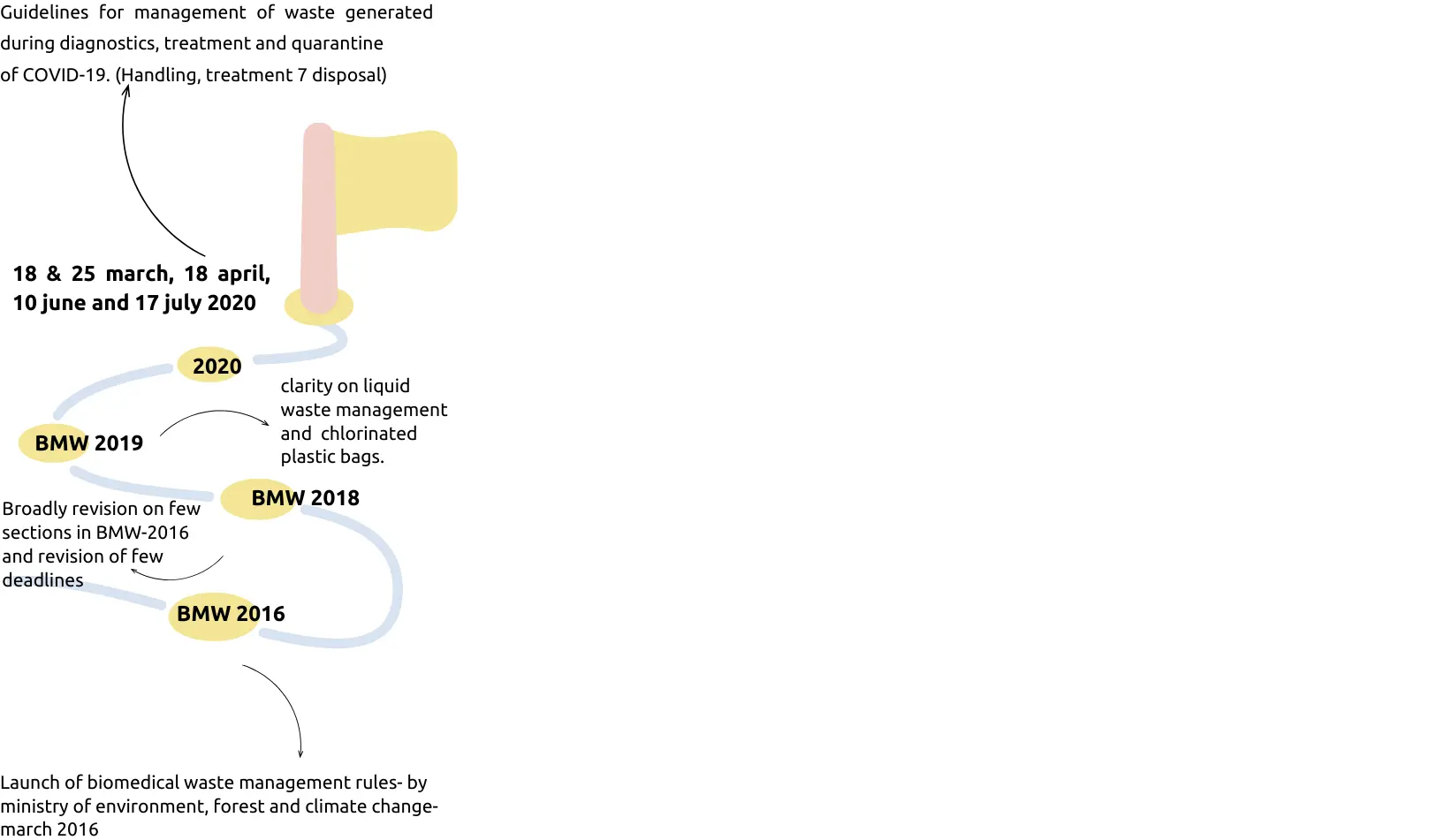
BMW Milestones
Uniform guidelines and code of practice for managing BMW
- Hospitals, nursing homes, clinics, dispensaries
- Veterinary institutions, animal houses
- Pathological laboratories, blood banks
- Ayush hospitals, Clinical establishments
- Research or educational institutions
- Health camps, medical or surgical camps, vaccination camps, blood donation camps
- First aid rooms of schools
- Forensic laboratories and research labs
BMW rules
Remember that the BMW rules of 2016 do not apply to:
1. Radioactive waste, Atomic Energy Act, 1987
The management of radioactive waste in India is regulated by the Atomic Energy Act of 1962, which was amended in 1987. The Act aims to regulate the development, control, and use of atomic energy. It sets the guidelines and provisions for the safe handling, storage, transportation, and disposal of radioactive materials and waste generated from nuclear facilities, medical institutions, and industrial applications. The 1987 amendment specifically addresses safety measures, licensing, and penalties associated with the misuse or mishandling of radioactive substances. This legislation ensures that activities related to atomic energy comply with stringent safety protocols to protect public health and the environment from potential hazards associated with radioactive waste.
2. Hazardous chemical rules, 1989
The Hazardous Chemical Rules of 1989 in India are legislative guidelines formulated under the Environment Protection Act of 1986. These rules were established to regulate and manage hazardous chemicals, ensuring their safe handling, transport, storage, and disposal. The rules aim to prevent and reduce the risks posed by hazardous chemicals to human health and the environment. They outline stringent measures for the identification, categorization, labelling, packaging, and transportation of such substances. Additionally, these rules mandate the creation of safety data sheets (SDS) and emergency response plans for hazardous chemical-related incidents, emphasizing the importance of employee training and public awareness regarding these materials& and potential risks. The Hazardous Chemical Rules play a pivotal role in minimizing hazards associated with the use and management of dangerous chemicals across various industrial sectors.
3. Solid wastes covered under MSW, rule, 2000
The Municipal Solid Wastes (Management and Handling) Rules, 2000 in India encompass various types of solid waste generated across different sectors. It covers biodegradable waste, comprising organic materials like kitchen waste, garden residue, and biodegradable packaging. Non-biodegradable waste, including plastics, metals,glass, and rubber, is also regulated under these rules. Additionally, it addresses domestic hazardous waste such as used batteries, paints, e-waste, and discarded medicines.
Construction and demolition waste, bulk waste like furniture and appliances, and healthcare waste from medical facilities, including biomedical waste, are among the categories managed through these regulations. The rules aim to streamline the segregation, collection, and appropriate disposal of these solid wastes to promote environmentally sustainable waste management practices.
4. Lead-acid batteries, batteries rule, 2001
The Batteries (Management and Handling) Rules, 2001 in India govern the management of lead acid batteries and other types of batteries to mitigate environmental hazards associated with their disposal. Lead acid batteries, commonly used in automobiles and industrial applications, fall under these regulations due to their potential environmental impact from lead and acid content. The rules specify guidelines for the collection, recycling, and safe disposal of used batteries. They aim to ensure proper handling to prevent soil and water contamination caused by lead leaching and acid spillage from discarded batteries. These rules enforce responsibilities on battery manufacturers, dealers, and consumers to facilitate the safe disposal and recycling of lead-acid batteries, minimizing their adverse effects on the environment.
5. Hazardous waste management handling & transboundary movement rules, 2008.
The Hazardous Waste (Management, Handling, and Transboundary Movement) Rules of 2008 in India are designed to regulate and manage hazardous waste to prevent adverse effects on human health and the environment.
These rules provide a comprehensive framework for the proper handling, treatment, storage, and disposal of hazardous waste. They specify guidelines for the identification, categorization, and classification of hazardous waste, along with measures for its safe transportation and transboundary movement. The regulations outline responsibilities for generators, transporters, and operators of facilities dealing with hazardous waste to ensure compliance with safety standards and environmental protection. Additionally, the rules emphasize the establishment of treatment, storage, and disposal facilities for the effective management of hazardous waste and the implementation of environmentally sound practices throughout its lifecycle.
6. E-waste, E-waste rules, 2011
The E-Waste (Management and Handling) Rules of 2011 in India aim to address the growing environmental and health concerns associated with electronic waste (e-waste). These rules provide a regulatory framework for the proper management, handling, and disposal of e-waste to minimize its impact on the environment and human health. The guidelines focus on the safe dismantling and recycling of electronic products, including obsolete or end-of-life electrical and electronic equipment. The rules prescribe procedures for the collection, storage, transportation, and treatment of e-waste, emphasizing the need for authorized dismantlers and recyclers. Additionally, the E-Waste Rules promote awareness and establish responsibilities for manufacturers, consumers, and other stakeholders in the e-waste management chain.
The overarching goal is to encourage sustainable practices, resource recovery, and the reduction of adverse effects associated with the disposal of electronic waste.
7. Hazardous micro-organisms rules, 1989
The Hazardous Micro-organisms Rules of 1989 in India were established to regulate and ensure safety in handling microorganisms that have the potential to cause diseases in humans, animals, or plants. These rules focus on containment, handling, and disposal of hazardous microorganisms to prevent their accidental release into the environment. The guidelines outline safety measures, including protocols for handling and transporting these microorganisms, safety practices in laboratories, and the requirement of appropriate containment facilities to prevent the spread of such organisms. Additionally, these rules highlight the importance of reporting and managing incidents involving hazardous microorganisms to mitigate potential risks to public health and the environment.
Statutory requirements
The health care organization possess a NOC from state Pollution control board (PCB) for generating, storage and disposal of BMW.
BMW-2016 rule defines
- Occupier: Administrator of the health care facility.
- Operator: Runs the common treatment facility
- Prescribed authority: The state pollution control board in respect of a state and pollution.
- Control Committee: Respect of a union territory.
Note: Occupier or the administrator of the healthcare facility and the operator or the person who runs the common treatment facility should maintain records of recyclable wastes.
Salient features of amended BMW rules 2016
- Pre-treat laboratory, microbiological, blood samples and blood bags through disinfection or sterilization on site.
- Phase out the use of chlorinated plastic bags, gloves and blood bags within two years.
- Provides training to all its healthcare workers and immunizes all health workers regularly.
- Established a barcode system for bags or containers containing bio-medical waste for disposal.
- Bio-medical waste has been classified into 4 categories instead of 10 to improve the segregation of waste at source.
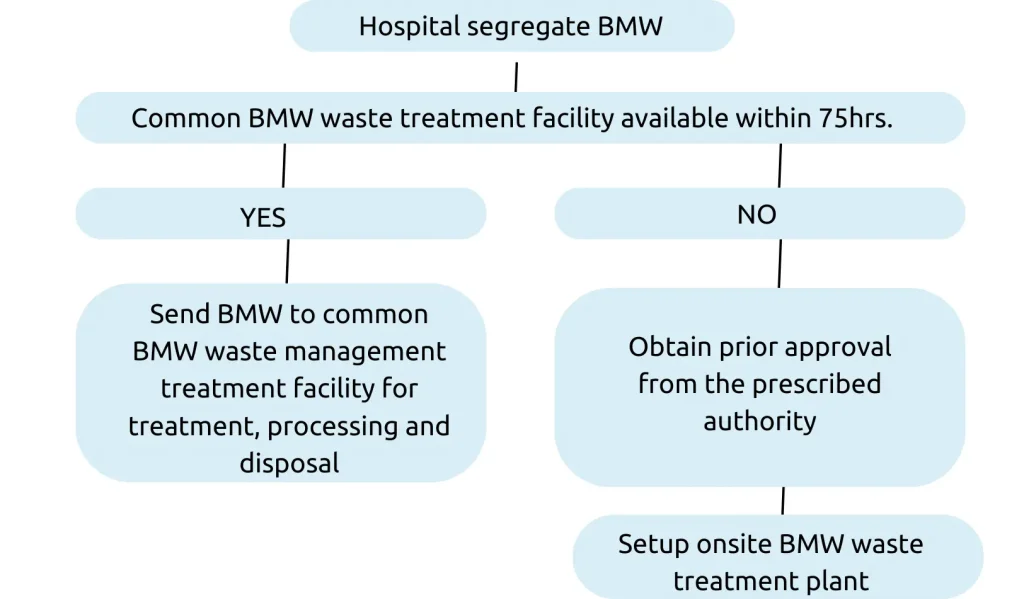
- No occupier shall establish a site treatment and disposal facility if services of a common biomedical waste treatment facility are available at a distance of seventy-five kilometres.
- Operator of a common biomedical waste treatment and disposal facility to ensure the timely collection (within 48 hours) of biomedical waste from the HCFs and assist the HCFs in the conduct of training.
- Annual report to be submitted in prescribed format on or before June 30 of every year.
Major accidents and its reporting
Report major accidents:
- While handling of bio-medical waste having potential to affect large masses of public and include toppling of the truck carrying bio-medical waste, release of bio-medical waste in any water body.
- Accidents caused by fire hazards, blasts during handling of biomedical waste the remedial action taken and the records relevant thereto, (including nil report) in form I to the prescribed authority and also along with the annual report.
- Exclude accidents like needle pick injuries, and mercury spills.
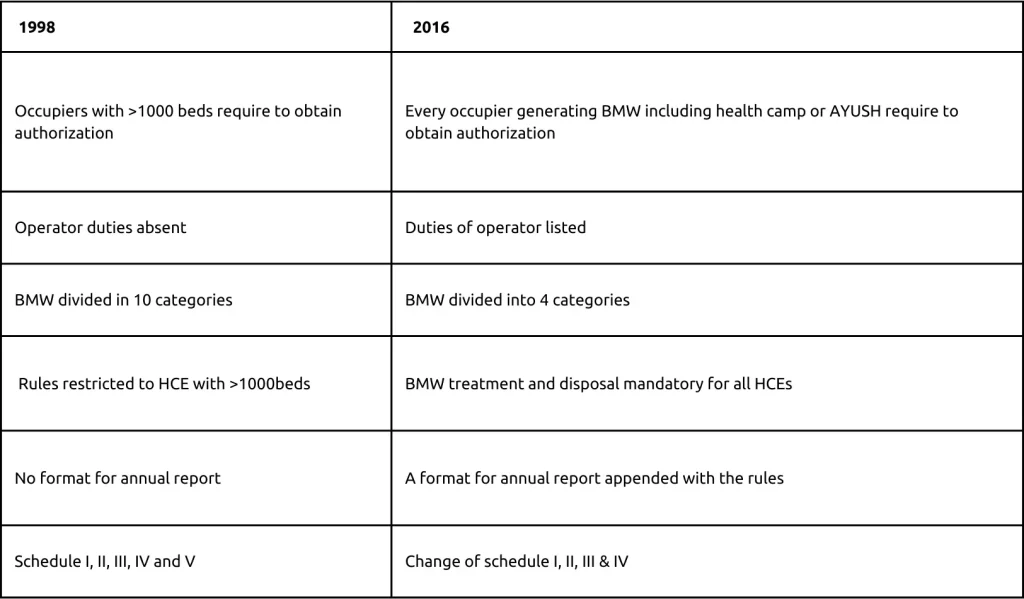
Major differences between BMW rules 1998 and 2016
Need for QR code system
1. Tracking of BMW
2. Daily check on Occupier & CBWTF
3. Preventing pilferage of BMW at Occupier and CBWTF
4. Identification of source of generation of BMW in case of improver disposal.
BMW rules amendment rules 2018
- Municipal waste handling should be substituted with solid waste management guidelines 2016.
- Hazardous waste (Management, Handling and transboundary Movement) Rules, 2008 should be substituted with hazardous and other wastes (Management and transboundary movement) rule, 2016.
- E-waste (Management and handling) rule, 2011- substitute with e-waste (Management) rules, 2016.
- All the healthcare facilites (any number of beds) shall make available the annual report on its website within a period of two years from the date of publication of bio-medical waste management (amendment) rule, 2018.
Against the category blue
Substitute blue coloured card boxes with puncture proof and leak proof boxes or containers with blue colored marking.

BMW rules amendment rules 2019 (19th feb 2019)
- The occupier of all bedded health care unit, shall maintain and update on a day to day basis the biomedical waste management register.
- All bedded healthcare units shall display the monthly record of waste disposal management on its website.
- Such health care facilities (irrespective of any number of beds), shall make the annual report available on its website before 19th march 2021.
- Health care facilities having less than ten beds shall have to comply with the output discharge standard for liquid waste by 31 December, 2019.

Penalty under the environment protection act, 1986
As per sec-15, sub-section-1
As per section-15, sub-section-2
Under Section 15, Sub-sections 1 and 2 of the Environment Protection Act, 1986, penalties are stipulated for violations of the Act’s provisions.
Sub-section 1 outlines penalties for non-compliance with the Act or its regulations, stating that contravention of any provision will result in a fine extending to Rs. 1,00,000 or imprisonment for a term not exceeding five years or both. Sub-section 2 specifies that if the offense continues after the initial conviction, an additional fine may be imposed for each day the offense persists, and this penalty may extend to Rs. 5,000 for every day during which the contravention continues after the first conviction.
Environment compensation As per Hon’ble NGT order
The term “environment compensation” refers to the monetary penalty or restitution imposed by the National Green Tribunal (NGT) as per its orders for violations against environmental laws or regulations. This compensation is aimed at mitigating environmental damage caused by an individual, entity, or industry. The NGT often issues directives or orders mandating compensation for environmental violations to deter future infractions and ensure environmental restoration or conservation. The specific amount or nature of compensation is determined by the NGT based on the severity of the violation and the impact on the environment.
Conclusion
The regulatory framework surrounding biomedical waste management in India, anchored by the Biomedical Waste Management Rules, 2016, stands as a robust structure designed to systematically manage medical waste.
These guidelines have undergone notable amendments to adapt to changing needs and technology. The rules emphasize stringent compliance, specifying duties for healthcare facilities, operators, and ensuring proper waste segregation, treatment, and disposal. The penalties outlined in the Environment Protection Act, 1986, and the concept of environmental compensation by the National Green Tribunal highlight the seriousness with which environmental violations are viewed. Overall, the framework not only aims to ensure compliance but also prioritizes environmental safety and public health in managing biomedical waste across the country.
Reference
Guideline for healthcare waste as per biomedical waste management rule, 2016.
Author
-

Chief Quality Officer, Infection Control Officer & Consultant Microbiologist, Caritas Hospital, Kottayam.