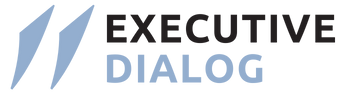Waste Management: The Indian Scenario
Read the Magazine in PDF
Abstract
The management of biomedical waste in India has undergone significant transformation over the years due to increasing concerns regarding its detrimental effects on public health and the environment. This historical overview delineates the evolution of biomedical waste management, from the nascent stages during the 1970s-1980s to the formulation of the Bio- Medical Waste (Management and Handling) Rules in 1998. Subsequent amendments and milestones in 2005, 2011, and 2016 marked pivotal moments, emphasizing the need for stringent guidelines, technological advancements, and public awareness campaigns. The current scenario underscores India’s stringent regulations for biomedical waste management, accentuating technological integration and public education to foster responsible waste handling practices. Technical guidelines have been established to address specific aspects, such as waste generated during COVID-19 treatment, environmental compensation, and verification protocols for waste treatment facilities. The imperative for effective biomedical waste management becomes apparent considering that only a fraction of hospital waste is categorized as hazardous, yet a significant gap exists in basic healthcare waste management across least developed countries. The healthcare sector substantial carbon footprint necessitates a transition towards sustainable waste management practices to mitigate environmental impact. However, challenges persist, notably regarding the interpretation of storage rules and the choice of containers for hazardous waste.
Debates continue between resource utilization, infection control, and sustainability.
Additionally, spill management protocols remain critical, requiring adherence to stringent disinfection procedures to contain and eliminate hazardous spills effectively.
In essence, while India has made substantial strides in biomedical waste management, challenges in interpretation, implementation, and compliance demand continued attention. Harmonizing regulations, enhancing infrastructure, and fostering sustainable waste management practices are pivotal for ensuring a safe and environmentally conscious healthcare system.
History of Biomedical Waste
The management of biomedical waste in India has evolved significantly over time due to growing concerns about its impact on public health and the environment. Here an overview of its history:
Early Years
Late 1980s: Incidents like the Bhopal Gas Tragedy in 1984 raised awareness about the hazardous impact of improper waste disposal.
Regulatory Framework: Bio-Medical Waste (Management and Handling) Rules, 1998: India introduced its first official guidelines for biomedical waste management.
These rules aimed to regulate the handling, treatment, and disposal of biomedical waste to minimize health and environmental risks.
Amendments
Over the years, amendments were made to these rules, such as the Biomedical Waste (Management and Handling) Amendment Rules in 2003 and subsequent revisions to enhance and strengthen the guidelines.
Key Milestones
- The introduction of colour-coded segregation and guidelines for waste segregation at the source was a significant development.
Further amendments emphasized the need for training, awareness programs.
Current Scenario
- Stringent Guidelines: India currently has stringent rules for biomedical waste management, focusing on segregation, collection, treatment, and safe disposal.
- Technological Integration: With advancements in technology, the emphasis is on innovative methods for waste treatment and disposal to reduce environmental impact.
- Public Awareness: Efforts are ongoing to raise awareness among healthcare personnel, waste handlers, and the public about proper waste management practices.
Technical guidelines
- Revision 5: Guidelines for handling treatment and disposal of waste generated during treatment, diagnostics and quarantine of COVID-19 patients.
- Guidelines for monitoring performance of CBWTFs by SPCBs/PCCs
- Revision 4: Guidelines for handling, treatment and disposal of waste generated during treatment, diagnostics and quarantine of COVID-19 patients.
- Advisory related to service of common biomedical waste treatment facilities to states
- Toolkit for biomedical waste management rules, 2016
- Guidelines for imposition of environmental compensation against HCFs and CBWTFs
- Guidelines for verification of two seconds residence time in secondary combustion chamber of the biomedical waste incinerator
- Environmentally sound management of mercury waste generated from healthcare facilities
- Guidelines for management of healthcare waste in healthcare facilities as per biomedical waste management rules, 2016
- Guidelines on the management of BMW generated during UIP
The need for biomedical waste management
- According to the WHO only about 15% of hospital waste falls into the hazardous category.
Yet a joint study by WHO and UNICEF found that only one in three HCFs in least developed countries have basic Health care waste management (HCWM).
Moving towards sustainability
- The health care sector is leading climate polluter, with healthcare’s climate footprint increasing from 4.4% of net global emissions in 2014 to 5.2% in 2019. Without action, healthcare emission could triple by 2050
- Minimum impact waste management-environment friendly waste treatment and disposal, yellow bag waste reduction, waste reduction and recycling, pharmaceutical and toxic chemical waste management, environmentally preferable purchasing etc.
- A 700 bedded hospital managing its waste wisely can reduce its carbon footprint by 1300MTCO2/y
- Indian medical sector would end up saving-272, 132MTCO2E emission in the environment, by recycling its plastic rather than incinerating it (2013).
Confusion on 48hour storage rule
- Most of the people are still following the 48h clause for the disposal of all BMW, as was mentioned in the 1998 rule i.e. ((5) No untreated bio-medical waste shall be kept stored beyond a period of 48hours).
- But in the 2016 rule, the clause was changed and only a few categories were put under this clause. (BMW rule 2016, say- (7) untreated human anatomical waste, animal anatomical waste, soiled waste and biotechnology waste shall not be stored beyond a period of 48hours)
- This was done to avoid wasteful disposal of sharps waste containers and container for glass waste.
Container for Sharps
- Most of the HCFs were using plastic cans in the hospitals as PPC
- Auditors expect use of proper white virgin PPCs
- Debate between resources utilization and sustainability Vs infection control
Spill Management protocol
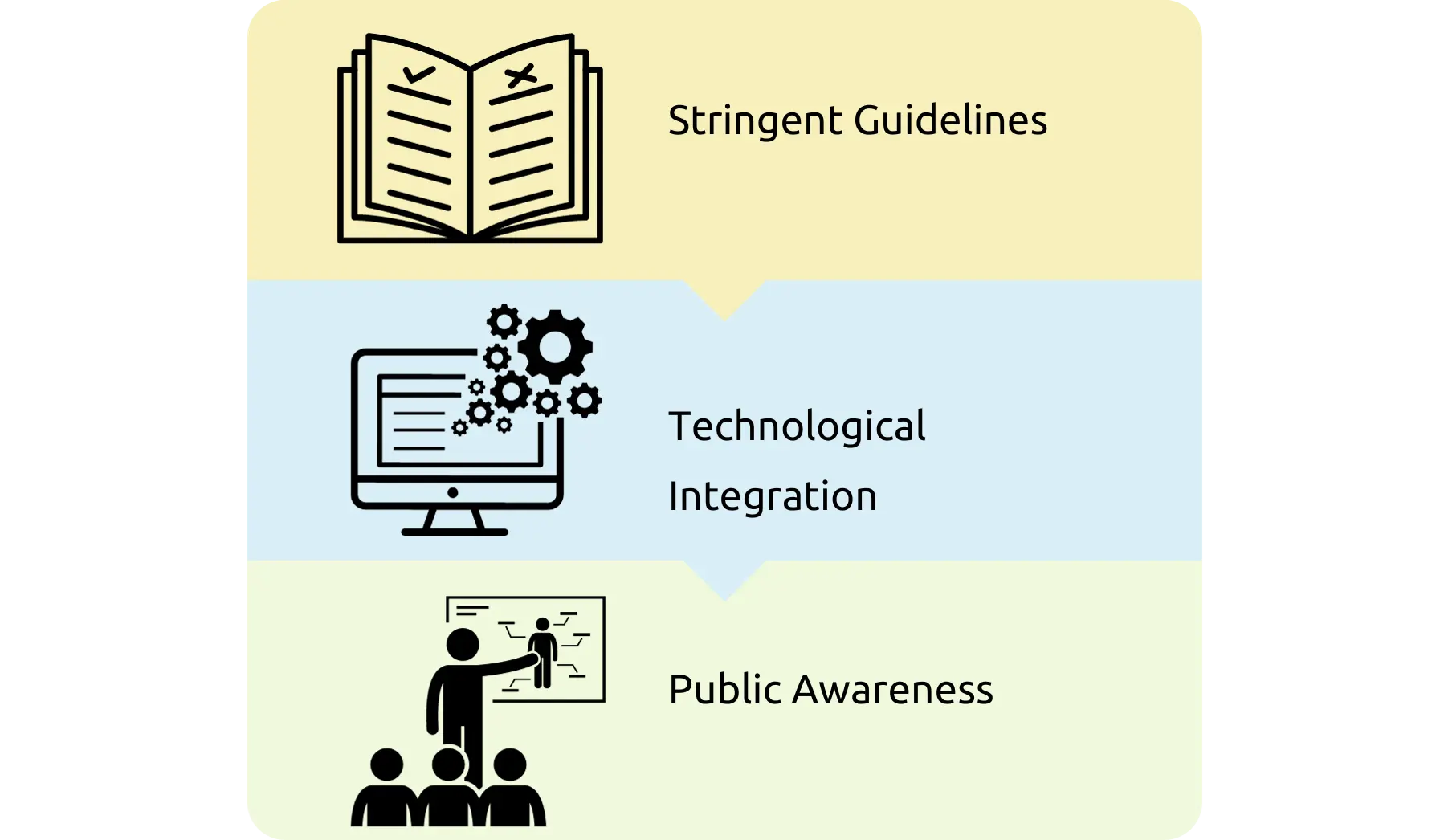
As per spill management protocol-CDC, WHO
- Wear appropriate PPE
- Confine the spill and wipe it up immediately with absorbent (paper) towels, cloths, or absorbent granules, and dispose as infectious waste.
- Clean thoroughly, using neutral detergent and warm water solution
- Disinfect by using facility-approved intermediate-level disinfectant. Typically, chlorine-based disinfectant at (1:100 or 1:10 dilution of 5% chlorine-bleach; depending on the size of the spill) are adequate. Moreover, it allows the disinfectant to remain on the surface for the required contact time (e.g., 10mins), and then rinse the area with clean water to remove the disinfectant residue (if required).
Other facts for spill management
For spill containing large amounts of blood or other body substances, workers should first remove visible organic matter with absorbent material (e.g disposable paper towels discarded into leak-proof, properly labelled containment) and then clean and decontaminate the area.
A recent study demonstrated that even strong chlorine solution (i.e., 1:10 dilution of chlorine bleach) may fail to totally inactivate high titers of virus in large quantities of blood, but in the absence of blood these disinfectants can achieve complete viral inactivation. this evidence supports the need to remove most organic matter from a large spill before final disinfection of the surface.
Challenges and Future Outlook
- Challenges persist, including ensuring compliance, infrastructure gaps, and monitoring practices across the country.
- Future strategies involve strengthening enforcement mechanisms, enhancing infrastructure, promoting research and innovation in waste management technologies, and continuous awareness programs.
Overall, India has made significant strides in addressing biomedical waste management, but ongoing efforts are essential to ensure effective implementation and compliance across all sectors involved in generating biomedical waste.
Conclusion
In conclusion, India’s trajectory in managing biomedical waste reflects an evolving commitment to public health and environmental welfare. The historical progression from initial guidelines to the present stringent regulations demonstrates the nations endeavor to address the complexities of waste management in healthcare settings. However, despite the advancements and robust guidelines, challenges persist. Clarification and uniform interpretation of storage rules, sustainable container choices, and effective spill management
protocols remain areas requiring attention and resolution. The imperative need to bridge the gap between guidelines and implementation is evident, emphasizing the importance of enhanced infrastructure, standardized practices, and continual education and awareness programs. Moving forward, a collaborative effort among regulatory bodies, healthcare institutions, and stakeholders is crucial to foster sustainable biomedical waste management practices. Aligning policies with practical application, harnessing technological innovations, and fostering a culture of environmental responsibility will be pivotal in ensuring a safe, sustainable, and environmentally conscious healthcare ecosystem in India.